Some of the fuctionalized allylsilanes obtained through silylcupration of allenes contain a nucleophilic allylsilane unit and an electrophilic moiety in the same molecule, which make them interesting synthons in anulation processes leading to five-, six- and seven-membered rings of structures found in some natural products.
Acc. Chem. Res., 2004, 37, 817-825
Thus, we can obtain methylenecylopentanols in a very stereoselective manner starting from allylsilyl aldehydes or ketones.
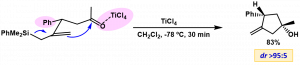
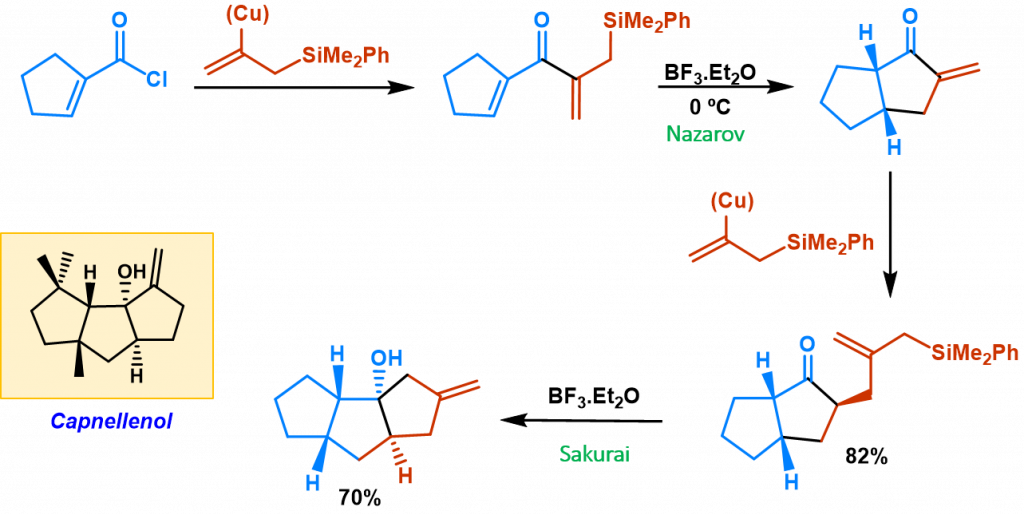
This methodology has succefully been applied to the synthesis of an analog of natural triquinane capnellenol throughout a four step sequence (silylcupration of allene, Nazarov cyclization, silylcupration and final acid-catalized cyclization).
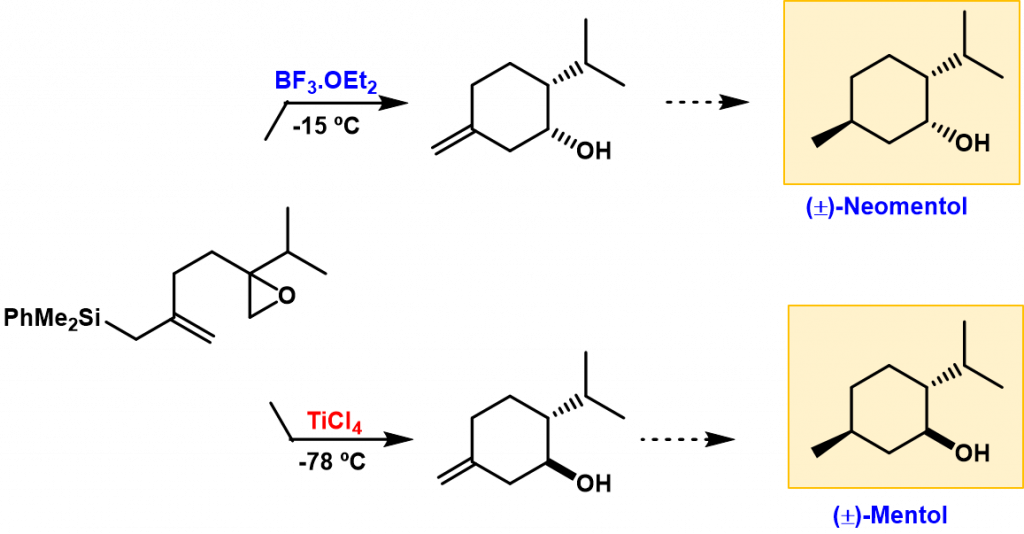
The cyclization of epoxyallylsilanes selectively provides methylenecyclohexanols through a rearrangement-cyclization process. We have been able to obtain both 1,6-syn and 1,6-anti cyclohexanol diastereoisomers in a selective fashion, through the right choice of the Lewis acid. This attractive feature has opened a route for the synthesis of both diastereomers of menthol from methylenecyclohexanols.
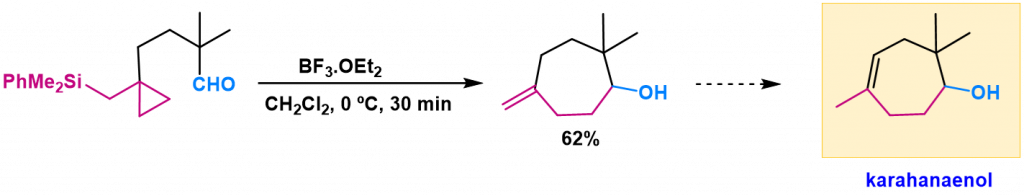
We have too developed an efficient and stereoselective method for cycloheptane ring formation from epoxy-cyclopropylsilanes through a tandem rearrangement-cyclization reaction. This methodology opens an easy entry to natural karahanaenol.