We have also been interested in the use of organosilanes for the synthesis of different sized heterocycles, especially medium sized oxa- and azacycles by silyl-Prins cyclization.
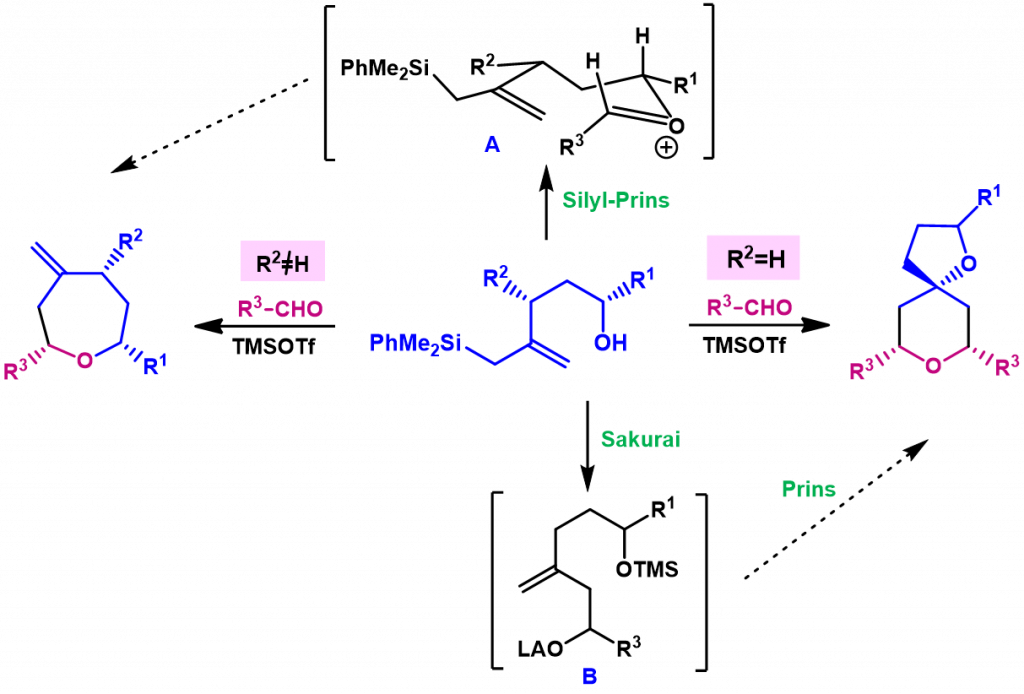
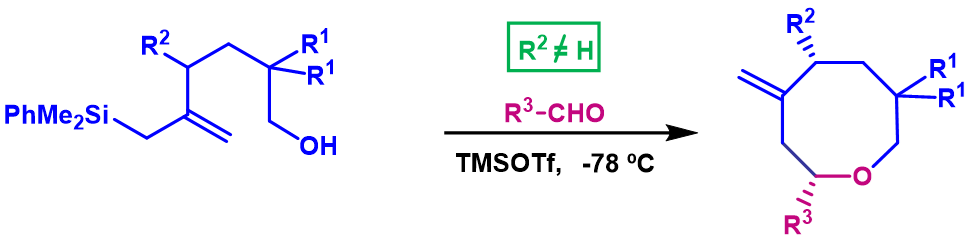
We have reported a remarkable substitution effect in the chemical pathway of the silyl-Prins cyclization of bis-homoallylic silyl alcohols. Apparently, the nature of the allylic substituent (R2=H or R2≠H) determines this change in the chemical outcome of the reaction, since alcohols having no substituents on the allylic position provide dioxaspirodecanes, from a tandem Sakurai-Prins cyclization, whereas alcohols with an allylic substituent R2≠H give selectively oxepanes, which correspond to a direct silyl-Prins cyclization.
This methodology has succefully been applied to the synthesis of an even more challenging target: 8-membered oxacycles. The cyclization of tris-homoallylic alcohols with aldehydes in the presence of TMSOTf proceeds in very high yield and with excellent stereocontrol to afford cis-2,5-disubstituted oxocanes. The reaction works well both for aromatic aldehydes (with electron donating or electron withdrawing susbstituents) and vinylic aldehydes.
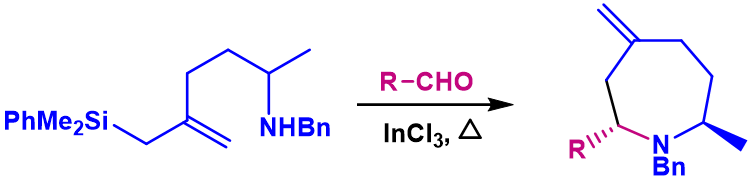
We have also succeeded, for the first time, in the synthesis of azepanes by silyl-azaPrins cyclization.